Which of the Following Statements About Dehydration Synthesis Is False
View the full answer. C Water is a polar molecule.

Solved Which Of The Following Statement About Dehydration Reactions Is False A Water Is Released During The Process B Small Sugar Chains Reply On Dehydration Reactions To Form Larger Polym C Amino Acids
D Covalent bonds are formed between the monomers.

. 134 A H 2 O is formed as the monomers are joined. E Salts readily dissolve in water. Finely divided palladium absorbs large volume of hydrogen gas.
Up to 24 cash back 14 Which of the following statements about dehydration synthesis is false. Group of answer choices Beta-pleated sheets are part of the secondary structure of proteins The nitrogenous bases of DNA are located on the inside because they are hydrophobic in character The peptide bond is formed by dehydration synthesis Alpha helices are stabilized by attraction between the amino acid R. A One monomer loses a hydrogen atom and the other loses a hydroxyl group.
Which of the above statement s is are correct. - Fat is a form of carbohydrate. One monomer loses a hydrogen atom and the other loses a hydroxyl group c.
D Water is formed as a part of a dehydration synthesis reaction. C Water molecules are formed by hydrolysis. D Animal digestive systems utilize this process to break down food.
C Water freezes from the top down. Water is formed by dehydration synthesis 2. Which of the following statements regarding carbon is.
B Water is a part of a dehydration reaction. D Animal digestive systems utilize this process to break down food. 14 Which of the following statements about dehydration synthesis is false.
E Water freezes from the top down. Hydrogen gas will not reduce heated aluminium oxide. B Electrons are shared between atoms of the joined monomers.
D Water is a part of a dehydration reaction. For each of the following statements about carbohydrates answer true or false and explain why. 3 Which of the following statements is false.
All of the above are true. A Water is a polar molecule. 4 Which of the following statements is FALSE.
B Water molecules are formed by hydrolysis. Electrons are shared between atoms of the joined monomers d. Covalent bonds are formed between the monomers b.
Water molecules form hydrogen bonds 4. C Water freezes from the top down. Glucose Fructose Sucrose Water.
Which of the following statements about dehydration synthesis is false. C Covalent bonds are formed between the monomers. A Salts readily dissolve in water.
Which of the following is a FALSE statement about carbohydrates. - Most enzymes are complex carbohydrates. B H2O is formed as the monomers are joined.
A Water is a polar molecule. Which of the following statements about dehydration synthesis is false-one monomer loses a hydrogen atom and the other loses a hydroxyl group-h2o is formed as the monomers are joined-Animal digestive systems utilize this process to break down food-covalent bonds are formed between the monomers. 15 Which of the following statements about dehydration synthesis is false.
Glycogen is a complex carbohydrate formed by dehydration synthesis of glucose molecules. E Water is a polar molecule. Dietary glucose is primarily used to drive our metabolic pathways that produce energy.
Course Title MGF 1001. B One monomer loses a hydrogen atom and the other loses a hydroxyl group. Which one of the following statements is FALSE.
A One monomer loses a hydrogen atom and the other loses a hydroxyl group. D Water molecules are formed by hydrolysis. B Water is a part of a dehydration synthesis reaction.
C Covalent bonds are formed between the monomers. A Salts readily dissolve in water. Atomic hydrogen is obtained by passing hydrogen through an electric arc.
134 Which of the following statements about dehydration synthesis is false. Pages 8 Ratings 100 1 1 out of 1 people found this document helpful. C Covalent bonds are formed between the monomers.
B Water molecules are formed by hydrolysis. D Water freezes from the top down. B Water molecules are formed by hydrolysis.
B Water is a part of a dehydration synthesis reaction. H2O is formed as the monomers are joined e. 27 Which of the following statements is FALSE.
Carbohydrates consist of monomers called simple sugars. School Florida International University. A One monomer loses a hydrogen atom and the other loses a hydroxyl group.
C H2O is formed as the monomers are joined. Water is a polar molecule 3. B H2O is formed as the monomers are joined.
A Salts readily dissolve in water. - The joining of two sugars to form a disaccharide is called a dehydration. D Animal digestive systems utilize this process to break down food.
E Water is a polar molecule. Which of the following statements regarding carbon is false A Carbon has a. Which of the following statements is false.
8 Identify the following reaction. Water can break ionic bonds 5.
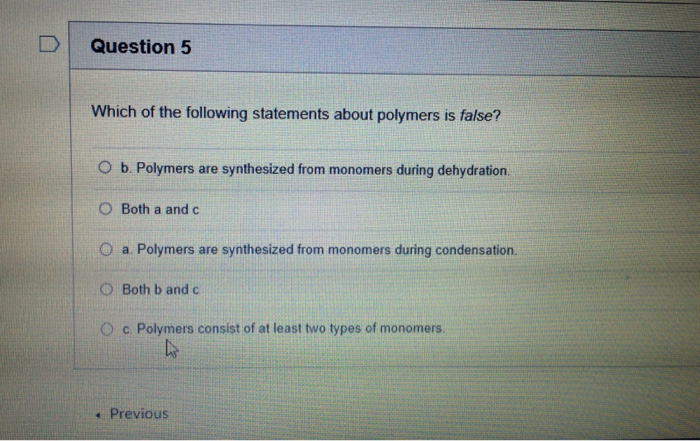
Solved D Question 5 Which Of The Following Statements About Chegg Com

Solved 1 Which Of The Following Statements Is False A Chegg Com
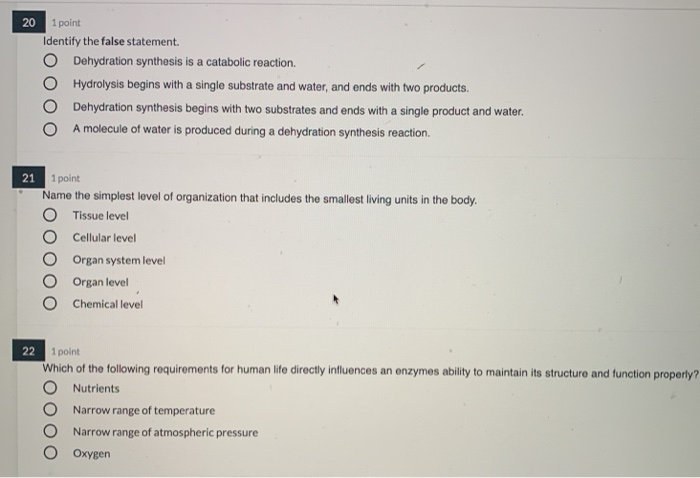
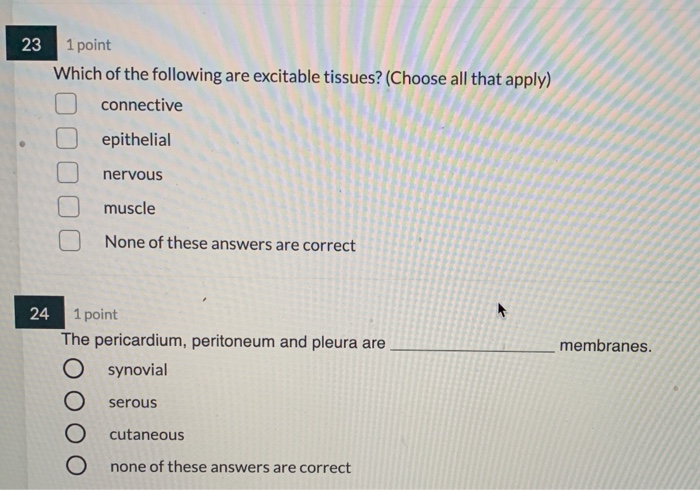
Comments
Post a Comment